1 Which One of the Following Is an Exothermic Process
Up to 256 cash back Which of the following processes is exothermic. Condensation of water vapor.

Endoexoreactions Exothermic Reaction Chemistry Lessons Teaching Chemistry
Pyrolysis is an endothermic process which takes place in the abundance of oxygen.

. Which one of the following is an exothermic reaction. What are the example of exothermic. The activation energy of an endothermic reaction _____.
Of gasoline Correct Answer. Which one of the following is an exothermic process. For 3 you are separating two Cl atoms.
Which one of the following is an exothermic process. Exothermic reaction is one that releases heat. E Both A and C Of the following which one is a state.
A ice melting B water freezing C boiling soup D Hydrochloric acid and barium hydroxide are mixed at 25 degree C. The reaction associated with DH degree f for an ionic compound the sublimation of dry ice CO2s the breaking of a CIÂCI bond the ionization of a lithium atom All of the above processes are exothermic. Correct option is A A large amount of heat is released because the hydration reaction of sulfuric acid is highly exothermic and dilution should always be performed by adding the acid to the water rather than the water to acid.
1 answer below. Boiling soup E Ammonium. A H B q c w D heat E none of the above Which one of the following conditions.
So it is exothermic. Condensation of a gas into a liquid. For 2 you are removing an electron.
Which one of the following is an exothermic process. Water evaporating ice melting boiling soup condensation of water vapor Ammonium thiocyanate and barium hydroxide are mixed at 25 C. Ii Exothermic first electron affinity - energy is released.
A ice melting B water evaporating C boiling soup D condensation of water vapor E Ammonium thiocyanate and barium hydroxide are mixed at 25 degree C. Reaction of water with quicklime - Heat is released. An exothermic process is any process that releases energy from the system to its surroundings.
A ice melting B water evaporating C boiling soup D condensation of water vapor E Ammonium thiocyanate and barium hydroxide are mixed at 25 C. Therefore it is an exothermic reaction. Catalytic cracking 2.
In beakers A and B an exothermic process has occurredII. Condensation of water vapor b. Which one of the following is an exothermic process.
Chemistry questions and answers. 12 Which one of the following is an exothermic process. In 4 you are adding an electron to an almost filled shell.
Which one of the following is an exothermic process. Methanol synthesis 3Oxidation of sulphur 4. Condensation of water vapor The specific heat capacity of.
The process of respiration is an exothermic reaction. Ice melting Ammonium thiocyanate and barium hydroxide are mixed at 25C. A ice melting B water evaporating C boiling soup D condensation of water vapor E Ammonium thiocyanate and barium hydroxide are mixed at 25 C.
Combustion is an exothermic process which takes place in the absence of oxygen. Which one of the following processes is exothermic. I Endothermic ionisation energy is required.
From among the following choose one which is not an exothermic process. During respiration breakdown of glucose in the presence of oxygen releases heat energy. Electronics Bazaar is one of best Online Shopping Store in India.
Exo means out and thermic means heat. Electronics Bazaar is one of best Online Shopping Store in India. Condensation of water vapor e.
Which one of the following processes is exothermic. Which of the following are exothermic processes. An exothermic process releases heat.
Pyrolysis is an exothermic process which takes place in the absence of oxygen. So it is endothermic. Which one of the following is an exothermic process.
Answer 20 Watch For unlimited access to Homework Help a Homework subscription is required. Options is. Evaporation of water - Heat needs to be absorbed for this reaction to take place.
A Electrolysis of water b Process of photosynthesis c Process of respiration. Dilution of an acid - When acid is diluted Heat is released. As hydration of sulfuric acid is thermodynamically favorable and the affinity of it for water is sufficiently strong.
Water evaporating condensation of water vapor temperature drops. Condensation of a gas into a liquid. I Reaction of water with quicklime ii Dilution of an acid Exothermic reactions are chemical reactions that release energy as heat or light.
A ice melting B water evaporating C boiling soup D condensation of water vapor E Ammonium thiocyanate and barium hydroxide are mixed at 25 C. Which one of the following is an endothermic process. An exothermic process is one that gives off heat.
Ammonium thiocyanate and barium hydroxide are mixed at 25 degree C. Sublimation of camphor crystals. Boiling a liquid melting a solid and converting a solid into a gas all require energy be put into the system.
Ammonium thiocyanate and barium hydroxide are mixed at 25C. Ammonia synthesis 5. I Reaction of water with quicklime ii Dilution of an acid iii Evaporation of water iv Sublimation of camphor crystals Answer.
Iii Endothermic higher electron affinity - energy is required. See also What Are The Best Wheelie Bikes. Pyrolysis is an endothermic process which takes place in the absence of oxygen.
An exothermic process releases heat. O water evaporating O boiling soup O condensation of water vapor. So Na s Na g is an endothermic reaction and you dont check that one.
Methanol synthesis 3Oxidation of sulphur 4. Ice melting water evaporating boiling soup condensation of water vapor Ammonium thiocyanate and barium hydroxide are mixed at 25 C. This heat is transferred to the surroundings.
Options is. Use that same kind of reasoning for 23 and 4. Hence both these processes produce cooling rather than heating.
Which one of the following is an exothermic process. Which one of the following is an exothermic process. 10 Which one of the following is an exothermic process.
Which one of the following is an exothermic process. So it is exothermic. Boiling soup E Ammonium thiocyanate and barium hydroxide are mixed at 25 C.
From among the following choose one which is not an exothermic process. Boiling a liquid melting a solid and converting a solid into a gas all require energy be put into the system. Which one of the following is an exothermic process.
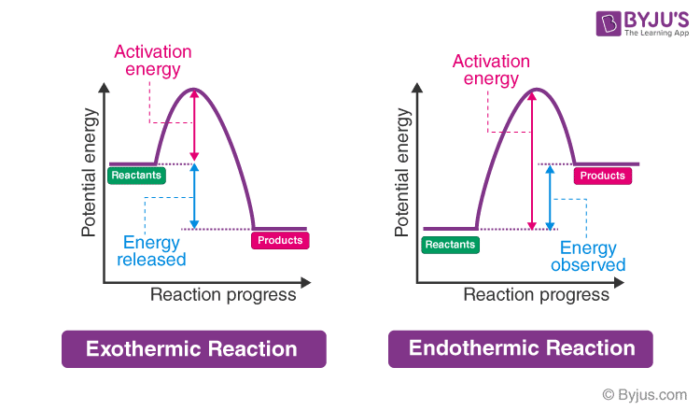
Endothermic And Exothermic Reaction Chemistry Quizizz

Exothermic Reaction Definition Equation And Examples

Exothermic Reaction Definition Equation And Examples

Endothermic Vs Exothermic Teaching Chemistry Chemistry Worksheets Science Teaching Resources

Endothermic And Exothermic Worksheets And Activities With Answers Exothermic Reaction How To Memorize Things Chemistry Lessons

Endothermic Vs Exothermic Reactions Chemtalk

Ncert Exemplar Mcq Which Of The Following Are Exothermic Processes
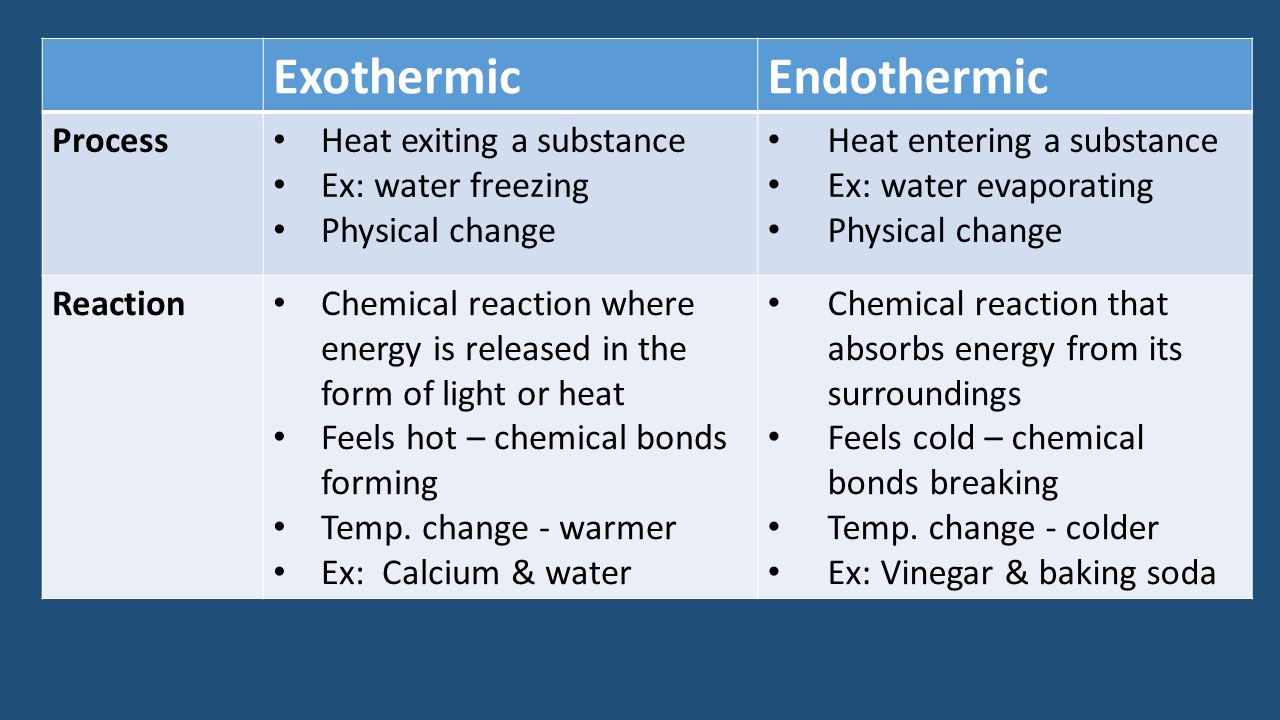
Endothermic Exothermic Processes Reactions Ppt Video Online Download

Comments
Post a Comment